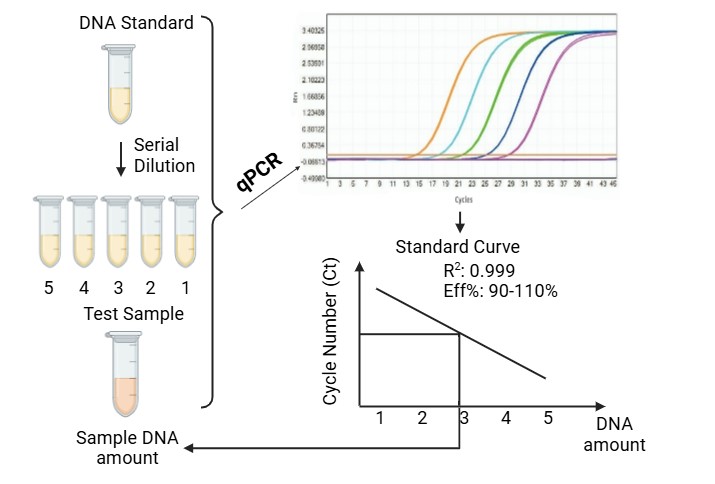
Creative Diagnostics, a reagent supplier and developer focused on biologics quality control, has announced the launch of its new Viral Nucleic Acid Residue Assay Kits (qPCR) to ensure the purity and safety of biopharmaceutical products by detecting and quantifying even minute levels of viral DNA and RNA contaminants. These kits can help manufacturers meet stringent regulatory requirements and quality control standards.
Creative Diagnostics' Viral Nucleic Acid Residue Assay Kits use state-of-the-art quantitative PCR (qPCR) technology that is highly sensitive and specific to reliably detect even trace amounts of viral nucleic acids. This capability is critical for maintaining the safety and efficacy of vaccines, gene therapies and other biological agents. In addition, these kits are designed to be easy to use, with reagents and detailed protocols for smooth integration into existing laboratory workflows.
Creative Diagnostics is committed to providing innovative solutions that address the critical needs of the biopharmaceutical industry. By providing rapid and accurate results, these kits help manufacturers meet stringent regulatory requirements and maintain strict quality control standards. Viral nucleic acid residue test kits (qPCR) are essential to the biopharmaceutical industry as they effectively monitor for viral contamination, protecting product integrity and patient safety and ensuring the highest quality of advanced therapeutic products.
For example, the ResDetFast™ E1A & SV40LTA DNA Residual Assay Kit (Cat. No. DDNAF-027) targets conserved E1A and SV40LTA sequences, paired with a ready-to-use reference for rapid and sensitive detection of residual E1A and SV40LTA DNA in biological products derived from HEK293T cells. HEK293T, a common expression vector in biopharmaceutical production, carries transformation sequences like Adv E1A and SV40 large T antigen, posing potential safety risks. When used with Creative Diagnostics Nucleic Acid Extraction or Purification Kit, this kit can detect trace amounts of E1A & SV40LTA DNA in as quickly as 1.5 hours, fulfilling regulatory requirements for sensitive detection of residual sequences in lentiviral vector preparations.
Adeno-Associated Virus (AAV), a member of the Parvoviridae family, is a widely used gene therapy vector. rAAV, a modified version of wild-type AAV, can be contaminated with rcAAV, a replication-competent form resulting from non-homologous recombination during production. The ResDetFast™ rcAAV-2/N Assay Kit (Cat. No. DDNAF-008), a quantitative fluorescence PCR assay targeting the GALV gene, enables rapid and sensitive detection of rcAAV in gene therapy products. Paired with a quantitative reference and compatible with Creative Diagnostics Nucleic Acid Extraction or Purification Kit, this kit can detect as few as 2 copies/μL of rcAAV within one and a half hours.
Creative Diagnostics' enhanced Viral Nucleic Acid Residue Assay Kits empower manufacturers to maintain the highest quality standards and ensure the safety of their products for patients worldwide. For more information on the new assay kits, please visit https://qbd.creative-diagnostics.com/products/viral-nucleic-acid-residue-assay-kits-qpcr-2793.html.
About Creative Diagnostics
Creative Diagnostics is a global leader in the development and manufacturing of innovative tools and reagents for bioprocess impurity analysis. The company offers a comprehensive portfolio of solutions to support researchers in the quality control of biologics and provides biopharmaceutical quality, purity and safety assays, analytical methods and applications for the biotechnology and biopharmaceutical industries.
Media Contact
Company Name: Creative Diagnostics
Contact Person: Thomas Schmitt
Email: Send Email
State: New York
Country: United States
Website: https://qbd.creative-diagnostics.com/