HYPER-H21-4 using DehydraTECH-CBD evidenced:
- Exceptional safety and tolerability
- Statistically significant lowering of 24-hour ambulatory blood pressure ("BP")
- BP lowered for the entire 5-week study duration
- BP lowered both for patients currently taking other antihypertensive drugs as well as patients not taking any other antihypertensive drugs
KELOWNA, BC / ACCESSWIRE / October 27, 2022 / Lexaria Bioscience Corp. (Nasdaq:LEXX) (Nasdaq:LEXXW) (the "Company" or "Lexaria"), a global innovator in drug delivery platforms is pleased to announce that human clinical study HYPER-H21-4 may be the world's first study to evidence a sustained drop in BP in normally active hypertensive patients following multiple weeks of oral cannabidiol ("CBD") therapy, using Lexaria's patented DehydraTECHTM-CBD capsule formulation.
The primary safety and efficacy objectives of study HYPER-H21-4 were successfully achieved. BP was significantly reduced by 2.5 weeks and was sustained over the full 5-weeks of dosing. These reductions in BP were achieved with zero serious adverse events being reported. Also, there were no adverse changes observed in liver enzymes; an important clinical safety biomarker of oral CBD therapy. Of note, these significant decreases in BP were achieved using relatively low doses of DehydraTECH-CBD as a direct result of the well-established drug delivery efficiencies of Lexaria's DehydraTECH technology.
In general, patients receiving placebo doses trended toward increases in BP during the period of the study compared to baseline, while the average BP measured by each of mean arterial BP ("MAP"), systolic BP ("SBP") and diastolic BP ("DBP") significantly decreased from baseline when dosed with DehydraTECH-CBD; and those decreases were maintained during the full 5 weeks of dosing. In fact, as shown in the table below, as much as a 7.01 mmHg differential in SBP was evidenced comparing the average SBP reduction from baseline on DehydraTECH-CBD to the average SBP increase from baseline upon cross-over on placebo following the second 2.5 weeks of dosing. Furthermore, there appeared to be a trend toward greater BP reduction over time and at the higher doses employed in the second 2.5 weeks of dosing; an important factor to be explored in future studies.
|
24-Hour BP Monitoring Change from Baseline |
Dose Period 1 First 2.5 Weeks (mmHg) |
Dose Period 2 Second 2.5 Weeks (mmHg) |
||
DehydraTECH-CBD (225 mg - 300 mg daily) |
Placebo |
DehydraTECH-CBD (375 mg - 450 mg daily) |
Placebo |
|
MAP |
-3.22 +/-0.90 P =0.002 |
+0.72 +/-0.78 P =1.000 |
-4.14 +/-0.69 P <0.001 |
-1.41 +/-0.91 P =0.387 |
SBP |
-4.76 +/-1.24 P =0.001 |
+0.94 +/-0.99 P =0.993 |
-5.60 +/-0.81 P <0.001 |
+1.41 +/-1.16 P =0.688 |
DBP |
-2.25 +/- 0.80 P =0.019 |
+0.67 +/-0.81 P =1.000 |
-3.25 +/-0.72 P <0.001 |
+1.30 +/-0.88 P =0.437 |
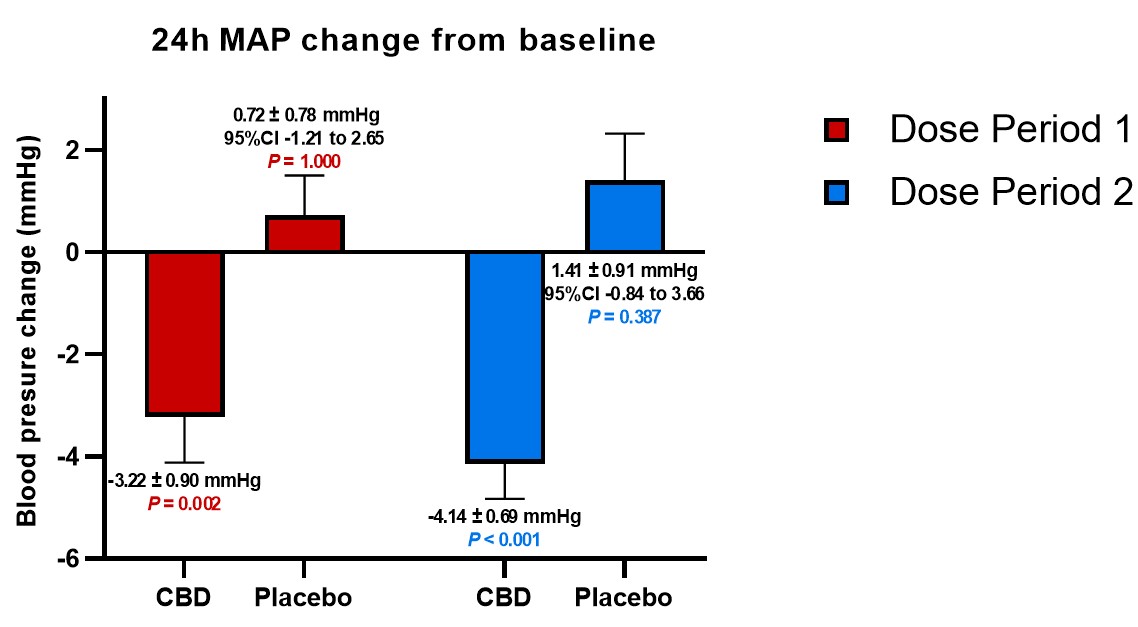
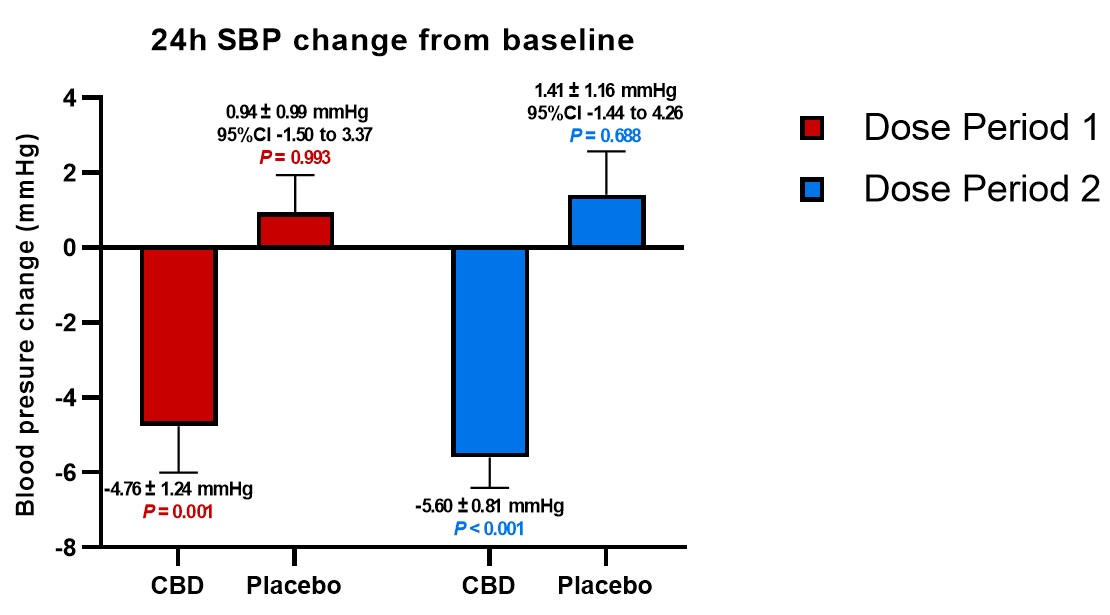
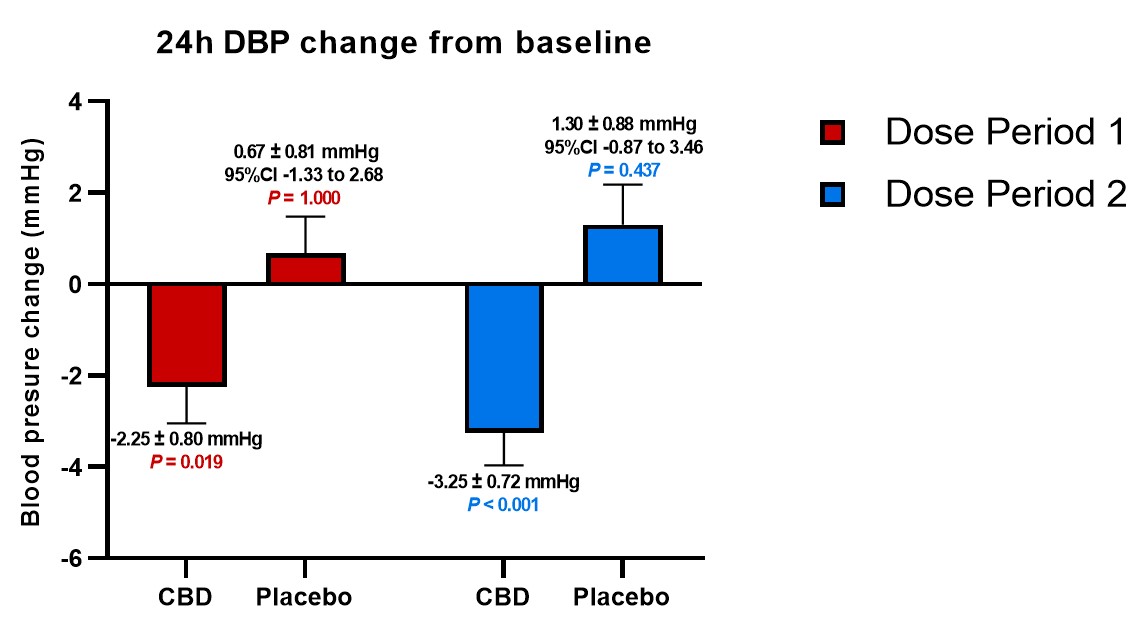
Other published research has established that, in order to reduce the collective risk of cardiovascular events such as myocardial infarction, stroke and congestive heart failure, reductions in BP of ~4.6 mmHg for SBP and ~2.2 mmHg for DBP are required. Lexaria's DehydraTECH-CBD BP reductions exceed these crucial levels.
Lexaria is aware of only a handful of other published research studies, mostly in young, healthy and normotensive volunteers, that have investigated whether a sustained decrease in resting BP is possible following multiple weeks of oral CBD dosing; none of which have been successful in achieving this. DehydraTECH-CBD is currently unique in its evidenced superior power to reduce BP over other oral CBD formulations.
"That we were able to lower blood pressure in our patient population over multiple weeks using DehydraTECH-CBD is an exceptional discovery, given that previous studies by others using other oral CBD formulations have failed to evidence this sustained benefit," said Chris Bunka, CEO of Lexaria Bioscience Corp. "DehydraTECH also demonstrated excellent safety and tolerability results and no adverse changes in liver enzymes throughout the study. Indeed, of the handful of minor, non-serious adverse events reported, there were nearly as many reported by those patients receiving placebo as those who received DehydraTECH-CBD. Consistent with Lexaria's often-mentioned de-risking strategy, this exceptional safety profile should prove beneficial as we prepare for our planned upcoming Phase Ib Investigational New Drug clinical study to be registered with the U.S. Food and Drug Administration."
Another important discovery from study HYPER-H21-4 was that the decreases in BP were similar in persons currently being treated with standard of care BP medications as in persons who were not undergoing any current standard of care BP treatment. This observation is suggestive that Lexaria's DehydraTECH-CBD has the potential to offer additive BP reduction benefits on top of any degree of improvements the standard of care medications achieved for those patients before entry into the study. This additive improvement as an adjunct therapy, together with the exceptional safety profile of DehydraTECH-CBD, could become a significant value enhancer should it eventually enter the marketplace as an approved hypertension treatment.
Additional study endpoint analyses as described in the complete study protocol are still underway and any relevant material findings will be reported upon in due course as these findings become available, currently expected during the remainder of 2022. The current findings will be submitted to an appropriate peer-reviewed clinical hypertension journal for possible publication.
ABOUT THE STUDY.
Study HYPER-H21-4 was a randomized, double-blinded, placebo-controlled, cross-over study that consisted of male and female volunteers between the ages of 40-70. Sixty-six (66) people were ultimately dosed to completion of the study, and they had documented or measured:
- elevated blood pressure (120/80 to 139/80 mmHg);
- mild (stage 1) hypertension (140/90 to 159/99 mmHg); or
- moderate (stage 2) hypertension (160/100 to 179/109 mmHg).
All study participants received DehydraTECH-CBD every day for a 5-week duration in the dose-escalating 2.5 week increments noted above. Upon cross-over and wash out, all 66 study participants also received the matching placebo for a 5-week duration following the study randomization schedule. Thirty-three (33) of these patients had been diagnosed with hypertension but were not being treated with any antihypertensive medications, while 33 patients had been diagnosed with hypertension and were receiving commonly used antihypertensive therapies including angiotensin-converting enzyme ("ACE") inhibitors with or without diuretics; or alternatively, ACE inhibitors with calcium channel blockers. The complete study protocol has already been published and is available at PubMed.
ABOUT LEXARIA BIOSCIENCE CORP.
Lexaria Bioscience Corp.'s patented drug delivery technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream by promoting more effective oral delivery. Since 2016, DehydraTECH has repeatedly demonstrated the ability to increase bio-absorption with cannabinoids, antiviral drugs, PDE5 inhibitors and more. DehydraTECH has also evidenced an ability to deliver some drugs more effectively across the blood brain barrier. Lexaria operates a licensed in-house research laboratory and holds a robust intellectual property portfolio with 27 patents granted and roughly 50 patents pending worldwide. For more information, please visit www.lexariabioscience.com.
CAUTION REGARDING FORWARD-LOOKING STATEMENTS
This press release includes forward-looking statements. Statements as such term is defined under applicable securities laws. These statements may be identified by words such as "anticipate," "if," "believe," "plan," "estimate," "expect," "intend," "may," "could," "should," "will," and other similar expressions. Such forward-looking statements in this press release include, but are not limited to, statements by the company relating the Company's ability to carry out research initiatives, receive regulatory approvals or grants or experience positive effects or results from any research or study. Such forward-looking statements are estimates reflecting the Company's best judgment based upon current information and involve a number of risks and uncertainties, and there can be no assurance that the Company will actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements. As such, you should not place undue reliance on these forward-looking statements. Factors which could cause actual results to differ materially from those estimated by the Company include, but are not limited to, government regulation and regulatory approvals, managing and maintaining growth, the effect of adverse publicity, litigation, competition, scientific discovery, the patent application and approval process, potential adverse effects arising from the testing or use of products utilizing the DehydraTECH technology, the Company's ability to maintain existing collaborations and realize the benefits thereof, delays or cancellations of planned R&D that could occur related to pandemics or for other reasons, and other factors which may be identified from time to time in the Company's public announcements and periodic filings with the US Securities and Exchange Commission on EDGAR. The Company provides links to third-party websites only as a courtesy to readers and disclaims any responsibility for the thoroughness, accuracy or timeliness of information at third-party websites. There is no assurance that any of Lexaria's postulated uses, benefits, or advantages for the patented and patent-pending technology will in fact be realized in any manner or in any part. No statement herein has been evaluated by the Food and Drug Administration (FDA). Lexaria-associated products are not intended to diagnose, treat, cure or prevent any disease. Any forward-looking statements contained in this release speak only as of the date hereof, and the Company expressly disclaims any obligation to update any forward-looking statements or links to third-party websites contained herein, whether as a result of any new information, future events, changed circumstances or otherwise, except as otherwise required by law.
INVESTOR CONTACT:
George Jurcic - Head of Investor Relations
ir@lexariabioscience.com
Phone: 250-765-6424, ext 202
SOURCE: Lexaria Bioscience Corp.
View source version on accesswire.com:
https://www.accesswire.com/722558/Lexarias-Human-Clinical-Hypertension-Study-a-Success













