New System Enhances Surgical Efficiency and Provides Versatile Solutions for Complex Spine Procedures
BELGRADE, MT / ACCESSWIRE / September 23, 2024 / Xtant Medical Holdings, Inc. (NYSE American:XTNT), a leading global medical technology company focused on the treatment of spinal disorders, is excited to announce the launch of the Cortera Posterior Fixation System, a comprehensive solution designed to streamline thoracolumbar fixation surgeries. Now available to surgeons across the United States through Xtant's commercial channels., Cortera enhances surgical workflows while delivering value across a wide spectrum of complex spinal procedures.
"The Cortera Posterior Fixation System is a significant addition to our growing fixation portfolio," said Sean Browne, President and CEO of Xtant Medical. "With innovative implants and multi-functional instruments, Cortera offers a safe, efficient solution for addressing complex spinal pathologies. This system simplifies procedures while maintaining consistent performance, allowing surgeons to focus on achieving optimal outcomes."
The launch of Cortera marks Xtant's second major product introduction this month, highlighting the company's commitment to expanding its portfolio with cutting-edge technologies. "By introducing new, innovative products, we are expanding our market opportunities," Browne added. "Customer feedback from our new products has been overwhelmingly positive, and the level of interest in Cortera has been very encouraging."
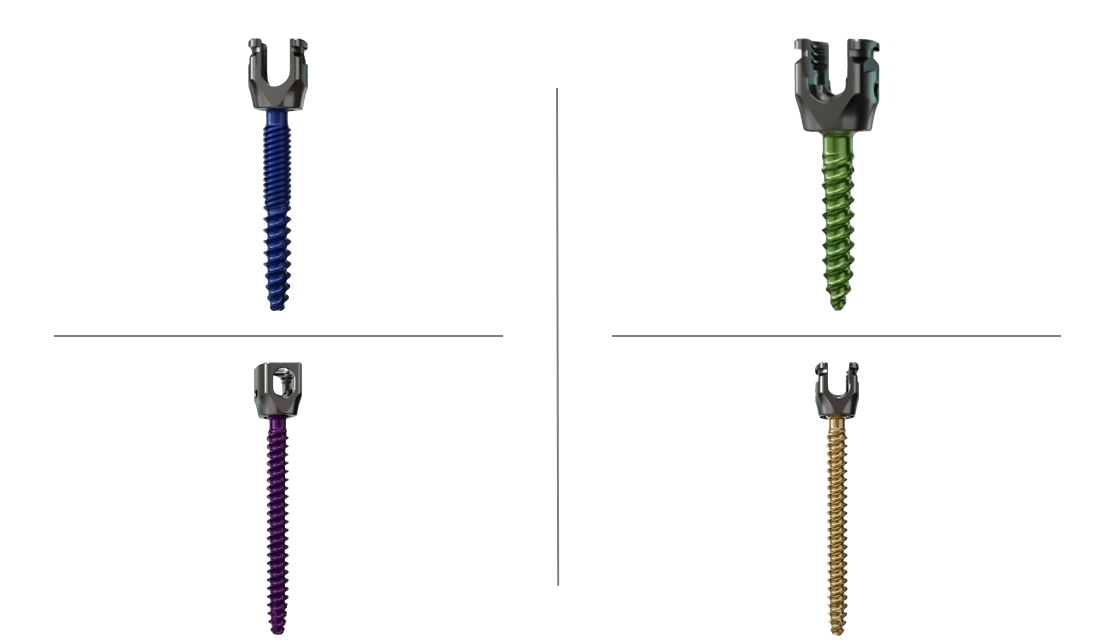
Key Features of the Cortera Posterior Fixation System:
- Simplifies both straightforward and complex posterior fixation procedures
- Dual Lead and Dual-to-Quad Lead screw thread options for greater versatility
- 5.5/6.0mm Ti/CoCr Rod System to enhance strength and stability
- Helical Flange design for secure fixation
- Universal S27 Hexalobe Drive for ease of use
![]()
The Cortera system addresses a broad range of patient needs, offering surgeons a reliable and
efficient tool for managing spinal deformities, fractures, and degenerative conditions. By improving workflow and minimizing operative challenges, the system enables more predictable surgical outcomes.
Xtant Medical will showcase the Cortera Posterior Fixation System at the NASS 2024 Annual Meeting, taking place from September 25-28, 2024, at McCormick Place in Chicago, IL. Attendees are invited to visit Booth #4402 to learn more about Cortera and Xtant Medical's Elevated Procedural Solutions.
For additional information about Cortera Posterior Fixation System, please visit https://xtantmedical.com/products/fixation/ or contact sales support at cs@xtantmedical.com or (888) 886-9354.
About Xtant Medical Holdings, Inc.
Xtant Medical's mission of honoring the gift of donation so that our patients can live as full and complete a life as possible, is the driving force behind our company. Xtant Medical Holdings, Inc. (www.xtantmedical.com) is a global medical technology company focused on the design, development, and commercialization of a comprehensive portfolio of orthobiologics and spinal implant systems to facilitate spinal fusion in complex spine, deformity and degenerative procedures. Xtant people are dedicated and talented, operating with the highest integrity to serve our customers.
The symbols ™ and denote trademarks and registered trademarks of Xtant Medical Holdings, Inc. or its affiliates, registered as indicated in the United States, and in other countries. All other trademarks and trade names referred to in this release are the property of their respective owners.
Cautionary Statement Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements that are predictive in nature, that depend upon or refer to future events or conditions, or that include words such as "intends," ‘‘expects,'' ‘‘anticipates,'' ‘‘plans,'' ‘‘believes,'' ‘‘estimates,'' "continue," "future," ‘‘will,'' "potential," "going forward," similar expressions or the negative thereof, and the use of future dates. Forward-looking statements in this release include the Company's expectations regarding the private placement, the timing for closing and the anticipated gross proceeds and use of net proceeds from the private placement. The Company cautions that its forward-looking statements by their nature involve risks and uncertainties, and actual results may differ materially depending on a variety of important factors, including, among others: risks and uncertainties surrounding the private placement; the Company's future operating results and financial performance; its ability to increase or maintain revenue; the Company's ability to become operationally self-sustaining and less reliant on third-party manufacturers and suppliers; risks associated with its acquisitions and the integration of those businesses; the Company's ability to implement successfully its future growth initiatives and risks associated therewith; possible future impairment charges to long-lived assets and goodwill and write-downs of excess inventory; the ability to remain competitive; the ability to innovate, develop and introduce new products and the success of those products; the ability to engage and retain new and existing independent distributors and agents and qualified personnel and the Company's dependence on key independent agents for a significant portion of its revenue; the effect of labor and hospital staffing shortages on the Company's business, operating results and financial condition, especially when they affect key markets; the effect of inflation, increased interest rates and other recessionary factors and supply chain disruptions; the effect of product sales mix changes on the Company's financial results; government and third-party coverage and reimbursement for Company products; the ability to obtain and maintain regulatory approvals and comply with government regulations; the effect of product liability claims and other litigation to which the Company may be subject; the effect of product recalls and defects; the ability to obtain and protect Company intellectual property and proprietary rights and operate without infringing the rights of others; risks associated with the Company's clinical trials; international risks; the ability to service Company debt, comply with its debt covenants and access additional indebtedness; the ability to obtain additional financing on favorable terms or at all; and other factors. Additional risk factors are contained in the Company's Annual Report on Form 10-K for the year ended December 31, 2023 filed with the SEC on April 1, 2024 and subsequent SEC filings by the Company. Investors are encouraged to read the Company's filings with the SEC, available at www.sec.gov, for a discussion of these and other risks and uncertainties. The Company undertakes no obligation to release publicly any revisions to any forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events, except as required by law. All forward-looking statements attributable to the Company or persons acting on its behalf are expressly qualified in their entirety by this cautionary statement.
Investor Relations Contact
Brett Maas
Managing Partner, Hayden IR
brett@haydenir.com
(646) 536-7331
SOURCE: Xtant Medical Holdings
View the original press release on accesswire.com













