- Preliminary data confirm Flow's ability to successfully measure neuro-effect of ketamine over 10 days -
- Results to be presented at PSYCH Symposium taking place in London on May 11, 2022 -
Kernel, a leader in non-invasive neuroimaging, and Cybin Inc. (NEO:CYBN) (NYSE American:CYBN), a biopharmaceutical company focused on progressing “Psychedelics to Therapeutics™,” are pleased to announce pilot results from a Cybin-sponsored feasibility study evaluating Kernel’s quantitative neuroimaging technology, Flow, to measure cortical hemodynamics while experiencing an altered state of consciousness.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220509005244/en/
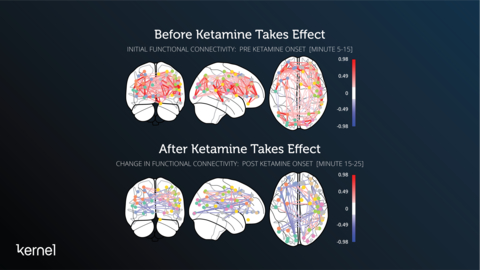
Top images: Johnson’s absolute functional connectivity for minutes 5-15 after he received the 57.75mg intramuscular injection of Ketamine. Bottom images: Johnson’s relative functional connectivity changes after the Ketamine took effect starting at minutes 15-25. (Graphic: Business Wire)
Preliminary data from the piloting suggested that ketamine-induced changes in functional connectivity persisted for several days after administration. Kernel Flow successfully measured the neuro-effect of ketamine over 11 days (baseline at Days 1-5, dosing at Day 6, follow-up at Days 7-11), and confirmed changes in functional connectivity that are consistent with current scientific research(1). The piloting was conducted to ensure the efficiency of the feasibility study design. Participants in the pilot received either a low dose of ketamine and/or a placebo while wearing the Flow headset.
“Kernel’s Flow technology is revolutionary in the field of brain imaging as it is the first easy-to-use wearable that can collect real-time brain activity before, during and after a psychedelic experience. Previously, studies had to rely on subjective patient reporting. By utilizing Flow, we will now be able to quantifiably gather real-time functional brain activity during our clinical and research studies evaluating psychedelic-based therapeutics,” said Doug Drysdale, Chief Executive Officer of Cybin. “Measuring where and how psychedelics work in the brain unlocks new frontiers of discovery in this space. Most importantly, Kernel Flow will enable us to measure the duration of effect during a psychedelic experience, which will be pivotal in developing the most accurate and effective treatment regimens for patients. This technology is precisely what we need to accelerate the development of psychedelics into therapeutics.”
“Kernel Flow is a groundbreaking neuroimaging technology that enables rigorous characterization and quantification of physiological processes in the human brain. We’re excited to report the pilot results of a longitudinally rich dataset of brain activity before, during a ketamine-induced altered-state experience, and after. The quality of the data recorded with Flow may lead to a better understanding of the neuro effects from psychedelics on the brain and help to advance these powerful new therapies for patients,” said Bryan Johnson, Founder and Chief Executive Officer of Kernel.
On March 31, 2022, Cybin and Kernel announced the initiation of the feasibility study and enrollment is ongoing.
The main objective of the feasibility study is to evaluate a participant’s experience wearing Kernel Flow while in an altered state of consciousness following the administration of ketamine. Participants will receive a low dose of ketamine and a placebo on separate visits while wearing the Flow headset. The system is equipped with hi-tech sensors to record brain activity and will report a participant’s experience using structured questionnaires and validated assessments during study visits and at follow-up. The four-week study will also evaluate brain activity before and after administering the study agents - low-dose ketamine or placebo.
The feasibility study received U.S. Food and Drug Administration (“FDA”) Investigational New Drug authorization in October 2021 and U.S. Institutional Review Board approval in January 2022.
Based on a partnership agreement between Cybin and Kernel dated January 11, 2021, Cybin will retain an exclusive interest in any innovations that are discovered or developed through its independent analysis of the study findings. Concurrently, Kernel will hold the same rights relating to its Kernel technology.
(1)Scheidegger et al 2012; Zacharias et al 2019; Li et al 2022
About Kernel Flow®
Kernel Flow is a wearable headset that measures brain activity by recording local changes in blood oxygenation. It is adjustable, can accommodate nearly anyone and is safe. Kernel Flow is groundbreaking neurotechnology because it reduces loud, expensive, and room-sized equipment to a head-worn apparatus while providing neural activity data of the highest possible optical quality. This combination has never existed in such a commercial and scalable device, all factors for why brain interfaces and neuroimaging technology has largely remained in academic labs or hospital basements. The entire system is the size and look of a bicycle helmet and could, in the future, be more broadly used for neuroscientific or physiological studies of brain activity during treatment.
About Cybin
Cybin is a leading ethical biopharmaceutical company, working with a network of world-class partners and internationally recognized scientists, on a mission to create safe and effective therapeutics for patients to address a multitude of mental health issues. Headquartered in Canada and founded in 2019, Cybin is operational in Canada, the United States, the United Kingdom and Ireland. The Company is focused on progressing Psychedelics to Therapeutics by engineering proprietary drug discovery platforms, innovative drug delivery systems, novel formulation approaches and treatment regimens for mental health disorders.
Cautionary Notes and Forward-Looking Statements
Certain statements in this press release constitute forward-looking information. All statements other than statements of historical fact contained in this press release, including, without limitation, statements regarding Cybin’s (“the Company”) future, strategy, plans, objectives, goals and targets, and any statements preceded by, followed by or that include the words “believe”, “expect”, “aim”, “intend”, “plan”, “continue”, “will”, “may”, “would”, “anticipate”, “estimate”, “forecast”, “predict”, “project”, “seek”, “should” or similar expressions or the negative thereof, are forward-looking statements. Forward-looking statements in this news release include statements regarding the Company’s proprietary drug discovery platforms, innovative drug delivery systems, novel formulation approaches and treatment regimens, the Company’s anticipated results using the Flow technology, and the ability of Flow to potentially accelerate the development of psychedelics into therapeutics.
These forward-looking statements are based on reasonable assumptions and estimates of management of the Company at the time such statements were made. Actual future results may differ materially as forward-looking statements involve known and unknown risks, uncertainties, and other factors which may cause the actual results, performance, or achievements of the Company to materially differ from any future results, performance, or achievements expressed or implied by such forward-looking statements. Such factors, among other things, include: implications of the COVID-19 pandemic on the Company’s operations; fluctuations in general macroeconomic conditions; fluctuations in securities markets; expectations regarding the size of the psychedelics market; the ability of the Company to successfully achieve its business objectives; plans for growth; political, social and environmental uncertainties; employee relations; the presence of laws and regulations that may impose restrictions in the markets where the Company operates; and the risk factors set out in the Company's management's discussion and analysis for the period ended December 31, 2021 and the Company's listing statement dated November 9, 2020, which are available under the Company's profile on www.sedar.com and with the U.S. Securities and Exchange Commission on EDGAR at www.sec.gov. Although the forward-looking statements contained in this news release are based upon what management of the Company believes, or believed at the time, to be reasonable assumptions, the Company cannot assure shareholders that actual results will be consistent with such forward-looking statements, as there may be other factors that cause results not to be as anticipated, estimated or intended. Readers should not place undue reliance on the forward-looking statements and information contained in this news release. The Company assumes no obligation to update the forward-looking statements of beliefs, opinions, projections, or other factors, should they change, except as required by law.
Cybin makes no medical, treatment or health benefit claims about Cybin’s proposed products. The FDA, Health Canada or other similar regulatory authorities have not evaluated claims regarding psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds. The efficacy of such products has not been confirmed by approved research. There is no assurance that the use of psilocybin, psychedelic tryptamine, tryptamine derivatives or other psychedelic compounds can diagnose, treat, cure or prevent any disease or condition. Rigorous scientific research and clinical trials are needed. Cybin has not conducted clinical trials for the use of its proposed products. Any references to quality, consistency, efficacy and safety of potential products do not imply that Cybin verified such in clinical trials or that Cybin will complete such trials. If Cybin cannot obtain the approvals or research necessary to commercialize its business, it may have a material adverse effect on Cybin’s performance and operations.
Neither the Neo Exchange Inc. nor the NYSE American LLC stock exchange have approved or disapproved the contents of this news release and are not responsible for the adequacy and accuracy of the contents herein.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220509005244/en/
Contacts
Leah Gibson
Vice President, Investor Relations & Strategic Communications
Cybin Inc.
leah@cybin.com
Kimberly Ha
press@kernel.com













