NASDAQ Co. Focused on CRISPR Gene Editing Technology from the Broad Institute of MIT and Harvard: Aridis Pharmaceuticals, Inc. (Stock Symbol: ARDS)
Aridis Pharmaceuticals, Inc. (OTCMKTS:ARDS)
We expect to maintain a high pace of execution and to position the company for an outstanding 2021”
LOS GATOS, CALIFORNIA, UNITED STATES, June 10, 2021 /EINPresswire.com/ -- NASDAQ Co. Focused on CRISPR Gene Editing Technology from the Broad Institute of MIT and Harvard: Aridis Pharmaceuticals, Inc. (Stock Symbol: ARDS)— Vu Truong, Ph.D., Chief Executive Officer of ARDS
Proprietary Products Currently in Clinical Trials
- Multiple Monoclonal Antibody Compounds Targeted to Key Infections.
- Specific Treatments for the Emerging Covid-19 Mutated Variants.
- Licensed CRISPR Gene Editing Technology from the Broad Institute of MIT and Harvard.
- ʎPEX Out-Licensing and Product Discovery Agreement with Kermode Biotechnologies, Inc. on Vaccines and mAbs for Zoonotic Viruses.
- Patients Currently in Ongoing Global Clinical Trials up to Phase 3.
Aridis Pharmaceuticals, Inc. (NASDAQ: ARDS) discovers and develops anti-infectives to be used as first-line treatments to combat antimicrobial resistance (AMR) and viral pandemics. ARDS is utilizing its proprietary ʎPEX and MabIgX® technology platforms to rapidly identify rare, potent antibody-producing B-cells from patients who have successfully overcome an infection, and to rapidly manufacture monoclonal antibody (mAbs) for therapeutic treatment of critical infections. These mAbs are already of human origin and functionally optimized by the natural human immune system for high potency. Hence, they are already fit-for-purpose and do not require further engineering optimization to achieve full functionality.
ARDS has generated multiple clinical stage mAbs targeting bacteria that cause life-threatening infections such as ventilator associated pneumonia (VAP) and hospital acquired pneumonia (HAP), in addition to preclinical stage antibacterial and antiviral mAbs. The use of mAbs as anti-infective treatments represents an innovative therapeutic approach that harnesses the human immune system to fight infections and is designed to overcome the deficiencies associated with the current standard of care which is broad spectrum antibiotics. Such deficiencies include, but are not limited to, increasing drug resistance, short duration of efficacy, disruption of the normal flora of the human microbiome and lack of differentiation among current treatments.
ARDS is NASDAQ listed with an attractive share structure of only 11,232,921 shares outstanding.
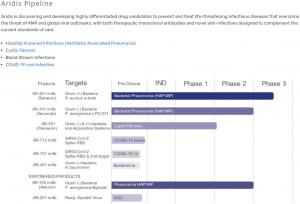
The mAb portfolio is complemented by a non-antibiotic novel mechanism small molecule anti-infective candidate being developed to treat lung infections in cystic fibrosis patients. The ARDS pipeline is highlighted below:
AR-301 (VAP). AR-301 is a fully human IgG1 mAb currently in Phase 3 clinical development targeting gram-positive Staphylococcus aureus (S. aureus) alpha-toxin in VAP patients.
AR-101 (HAP). AR-101 is a fully human immunoglobulin M, or IgM, mAb in Phase 2 clinical development targeting Pseudomonas aeruginosa (P. aeruginosa) liposaccharides serotype O11, which accounts for approximately 22% of all P. aeruginosa hospital acquired pneumonia cases worldwide.
AR-501 (cystic fibrosis). AR-501 is an inhaled formulation of gallium citrate with broad-spectrum anti-infective activity being developed to treat chronic lung infections in cystic fibrosis patients. This program is currently in a Phase 2a clinical study in healthy volunteers and CF patients.
AR-401 (blood stream infections). AR-401 is a fully human mAb preclinical program aimed at treating infections caused by gram-negative Acinetobacter baumannii.
AR-701 (COVID-19). AR-701 is a cocktail of fully human mAbs discovered from convalescent COVID-19 patients that are directed at multiple envelope proteins of the SARS-CoV-2 virus.
AR-712 (COVID-19). AR-712 is cocktail of two fully human IgG1 mAbs, AR-711 and AR-713, that are directed against the receptor binding domain of the SARS-CoV 2 virus. AR-712 is being developed to treat non-hospitalized mild to moderate COVID-19 patients by inhalation using a nebulizer.
AR-201 (RSV infection). AR-201 is a fully human IgG1 mAb directed against the F-protein of diverse clinical isolates of respiratory syncytial virus (RSV). This program is licensed exclusively to the Serum Institute of India.
- ARDS Announces First Quarter 2021 Update
On May 11thARDS reported financial and corporate results for the first quarter ended March 31, 2021. Highlights include:
Announced the addition of a second inhaled monoclonal antibody ("mAb") to neutralize newly emerging COVID-19 mutated variant to form a cocktail of two mAbs. The expansion of COVID virus strain coverage, combined with the product's self-administered, at-home treatment modality, further differentiates the company's AR-712 COVID treatment offering. A clinical Phase 1/2 study is expected to be launched in 2H 2021.
- Announced the addition of a second inhaled monoclonal antibody ("mAb") to neutralize newly emerging COVID-19 mutated variant to form a cocktail of two mAbs. The expansion of COVID virus strain coverage, combined with the product's self-administered, at-home treatment modality, further differentiates the company's AR-712 COVID treatment offering. A clinical Phase 1/2 study is expected to be launched in 2H 2021.
- Announced the preclinical development services support of the company's COVID mAb program from NIAID and the Coronavirus Immunotherapy Consortium (CoVIC).
- Licensed the CRISPR gene editing technology from the Broad Institute of MIT and Harvard. CRISPR is a component of the ʎPEXTM mAb discovery and production platform technology.
- Entered into a ʎPEX out-licensing and product discovery agreement with Kermode Biotechnologies, Inc. on vaccines and mAbs for zoonotic viruses, which are animal viruses that have the ability to infect humans and potentially lead to a viral pandemic.
- Continued enrolling global Phase 3 clinical trial of AR-301 in patients with ventilator associated pneumonia (VAP) including patients who presented with S. aureus VAP as a secondary infection to COVID-19. Interim futility analysis is expected in 2H 2021 and top-line data expected in 1H 2022.
- Executed a registered direct offering with gross proceeds of approximately $8.5 million in the fourth quarter of 2020 and approximately $7.0 million in March 2021.
As the pandemic has raged on this past year, with the virus evolving into more transmissible and lethal variants, ARDS has been able to adapt its mAb offering to expand strain coverage to all key SARS-COV-2 variants. ARDS also continued to optimize its versatile ʎPEX mAb discovery platform technology and entered into a partnership to develop mAbs and vaccines for viruses with pandemic potential.
COVID-19 Program Update
AR-712: During the quarter, AEDS announced the development of a highly potent fully human mAb against SARS-CoV-2 virus. AR-712 is a cocktail of two mAbs, AR-711 and AR-713, designed to lower the barrier to treatment coverage of non-hospitalized COVID-19 patients by using a convenient, self-administered inhaled dosage presentation. The two mAbs that comprise AR-712 were discovered from convalescent COVID-19 patients and target the receptor-binding domain (RBD) region of the spike protein of the original SARS-CoV2 virus and its newly emerging variants including the currently prevalent strain 'E484K' associated with the South Africa, Brazil, and Japan variants. The ARDS antibody also neutralizes the California strain, which harbors the L452R mutation.
In an animal challenge study with golden Syrian hamsters, inhaled AR-711 successfully eliminated all detectable SARS-CoV-2 virus at substantially lower doses than parenterally administered (injected) COVID-19 mAb. The AR-712 mAbs are engineered to be long-acting in blood for up to six to twelve months and are stabilized using a proprietary formulation designed to protect the mAbs from the physical stresses imparted by commercial nebulizer delivery devices on protein drugs. The potency of AR-712 and its direct delivery to the lungs by inhaled administration may facilitate significant dose sparing not achievable by parenteral administration. A proprietary formulation enables AR-712 to be deliverable using a variety of commercially available nebulizers that can be self-administered on an outpatient basis, thus lowering the barrier to COVID-19 therapeutic treatment. Clinical trials for AR-712 are expected to commence 2H 2021.
AR-701: During the quarter, ARDS continued to characterize this cocktail of fully human mAbs discovered from its in-house ʎPEX mAb discovery platform that is directed at multiple envelope proteins of the SARS-CoV-2 virus. AR-701 is intended to treat hospitalized, moderate to severe patients, which complements AR-712's focus on milder, non-hospitalized patients.
Clinical Program Update
AR-301: AR-301 is being evaluated in a Phase 3 clinical study as an adjunctive treatment to standard of care antibiotics in S. aureus infected ventilator associated pneumonia (VAP) patients. Thus far, the pace of the trial has continued to be impacted by the protracted COVID-19 pandemic. The Phase 3 interim futility analysis from the ongoing pivotal trial is now expected to be reported in 2H 2021 and top line data by 1H 2022. It's important to note that COVID-19 patients on prolonged mechanical ventilation in the intensive care unit (ICU) are prone to secondary infections (also called 'superinfections') by opportunistic pathogens such as bacteria. Superinfection is a reported complication in COVID-19 patients, which exacerbates morbidity and the rate of mortality. The AR-301 Phase 3 study allows for the enrollment of patients with baseline characteristics which are inclusive of certain COVID-19 patients. While AR-301 is not an agent to treat SARS-CoV-2 virus itself, it can potentially reduce the morbidity associated with secondary S. aureus pneumonia, which is a coronavirus complication and a contributing cause of death in such patients.
The trial is expected to enroll 240 patients at approximately 160 clinical centers in 22 countries. Participating clinical centers that are activated continue to follow standard stringent clinical protocols and procedures for critically ill VAP patients, as is standard in the U.S. and Europe. The trial represents the first ever Phase 3 superiority clinical study evaluating immunotherapy with a fully human monoclonal antibody to treat acute pneumonia in the intensive care unit setting. Details of the study can be viewed on www.clinicaltrials.gov using identifier NCT03816956.
AR-501: During the quarter, ARDS initiated the Phase 2a study to evaluate the safety, pharmacokinetic, and preliminary efficacy evaluation in cystic fibrosis (CF) patients. The Phase 2a is set to enroll 42 cystic fibrosis adult patients and is expected to complete enrollment by the end of 2021.
AR-501 is being developed in collaboration with the CF Foundation and has been granted Orphan Drug Designation (ODD), Fast Track and Qualified Infectious Disease Product (QIDP) designations by the FDA. In addition, the European Medicines Agency (EMA) granted ODD to AR-501. The original Phase 1/2a clinical trial was a randomized, double-blinded, placebo-controlled SAD and MAD trial investigating the safety and PK of inhaled AR-501 in healthy volunteers and cystic fibrosis patients with chronic bacterial lung infections. Details of the original Phase 1/2a clinical trial can be viewed on www.clinicaltrials.gov using identifier NCT03669614.
Corporate Update
A key development was the closing of a $7.0 million financing which occurred in March 2021. The proceeds from this registered direct offering strengthened the ARDS balance sheet during the first quarter to support the continued advancement of AR-301's Phase 3 VAP clinical trial, while allocating the requisite resources to AR-501's Phase 2b cystic fibrosis clinical trial and the ongoing development of novel COVID-19 therapies such as AR-712 and AR-401.
For additional information on Aridis Pharmaceuticals (ARDS) visit https://www.aridispharma.com.
DISCLAIMER: FrontPageStocks/CorporateAds.com (CA) is a third-party publisher and news dissemination service provider. FPS/CA is NOT affiliated in any manner with any company mentioned herein. FPS/CA is news dissemination solutions provider and are NOT a registered broker/dealer/analyst/adviser, holds no investment licenses and may NOT sell, offer to sell or offer to buy any security. FPS/CA’s market updates, news alerts and corporate profiles are NOT a solicitation or recommendation to buy, sell or hold securities. The material in this release is intended to be strictly informational and is NEVER to be construed or interpreted as research material. All readers are strongly urged to perform research and due diligence on their own and consult a licensed financial professional before considering any level of investing in stocks. All material included herein is republished content and details which were previously disseminated by the companies mentioned in this release or opinion of the writer. FPS/ CA is not liable for any investment decisions by its readers or subscribers. Investors are cautioned that they may lose all or a portion of their investment when investing in stocks. FPS/CA has been compensated $500 by a third party for dissemination of this Article.
Disclaimer/Safe Harbor:
These news releases and postings may contain forward-looking statements within the meaning of the Securities Litigation Reform Act. The statements reflect the Company’s current views with respect to future events that involve risks and uncertainties. Among others, these risks include the expectation that any of the companies mentioned herein will achieve significant sales, the failure to meet schedule or performance requirements of the companies’ contracts, the companies’ liquidity position, the companies’ ability to obtain new contracts, the emergence of competitors with greater financial resources and the impact of competitive pricing. In the light of these uncertainties, the forward-looking events referred to in this release might not occur.
SOURCE: CorporateAds.com
Dr. Vu L. Truong CEO and Founder
Aridis Pharmaceuticals, Inc.
+1 408-385-1742
email us here
Visit us on social media:
Facebook
LinkedIn
Aridis Pharmaceuticals Inc. Nasdaq: ARDS