GRENOBLE, France, Nov. 20, 2024 (GLOBE NEWSWIRE) -- Koelis, SAS (“Koelis” or the “Company”, www.koelis.com), a global leader and innovator in prostate care, announced today passing an important milestone demonstrating the value of its Koelis Trinity 3D Ultrasound Platform as the enabling technology for prostate focal therapy: The VIOLETTE multicentric phase II trial on Targeted Microwave Ablation has finalized enrollment, published interim results in the respected BJUI Compass and will be presented at the prestigious annual French Urology meeting in Paris this week.
Recent advances in imaging technology have helped reduce the overdiagnosis and overtreatment of prostate cancer. The use of prostate MRI prior to the so-called “fusion” biopsy guided by MRI/ultrasound fusion imaging has proven its ability to visualize and accurately describe cancerous lesions on the way to a more personalized treatment.
Thanks to the Koelis Trinity® fusion imaging system, a platform based on a disruptive technology called Organ-Based Tracking® (OBT-Fusion), which combines 3D ultrasound and MRI imaging by software fusion technology, clinicians can visualize the prostate in real-time and create a 3D map while recording the identified lesions.
Koelis’ OBT-Fusion® also enables the planning and guidance with high precision of treatment needles into targeted areas. Among possibilities, Koelis has chosen to evaluate the use of its technology in a minimally invasive approach called Targeted Microwave Ablation (TMA™), through a multicentric European Phase II trial called VIOLETTE (NCT04582656).
TMA stands out from other Focal Therapy options by the fact that it is a global strategy, including not only a specific ablation modality but also and more importantly a comprehensive guidance tool allowing to recall the location of the targeted biopsy as well as to visualise the MRI-visible tumour.
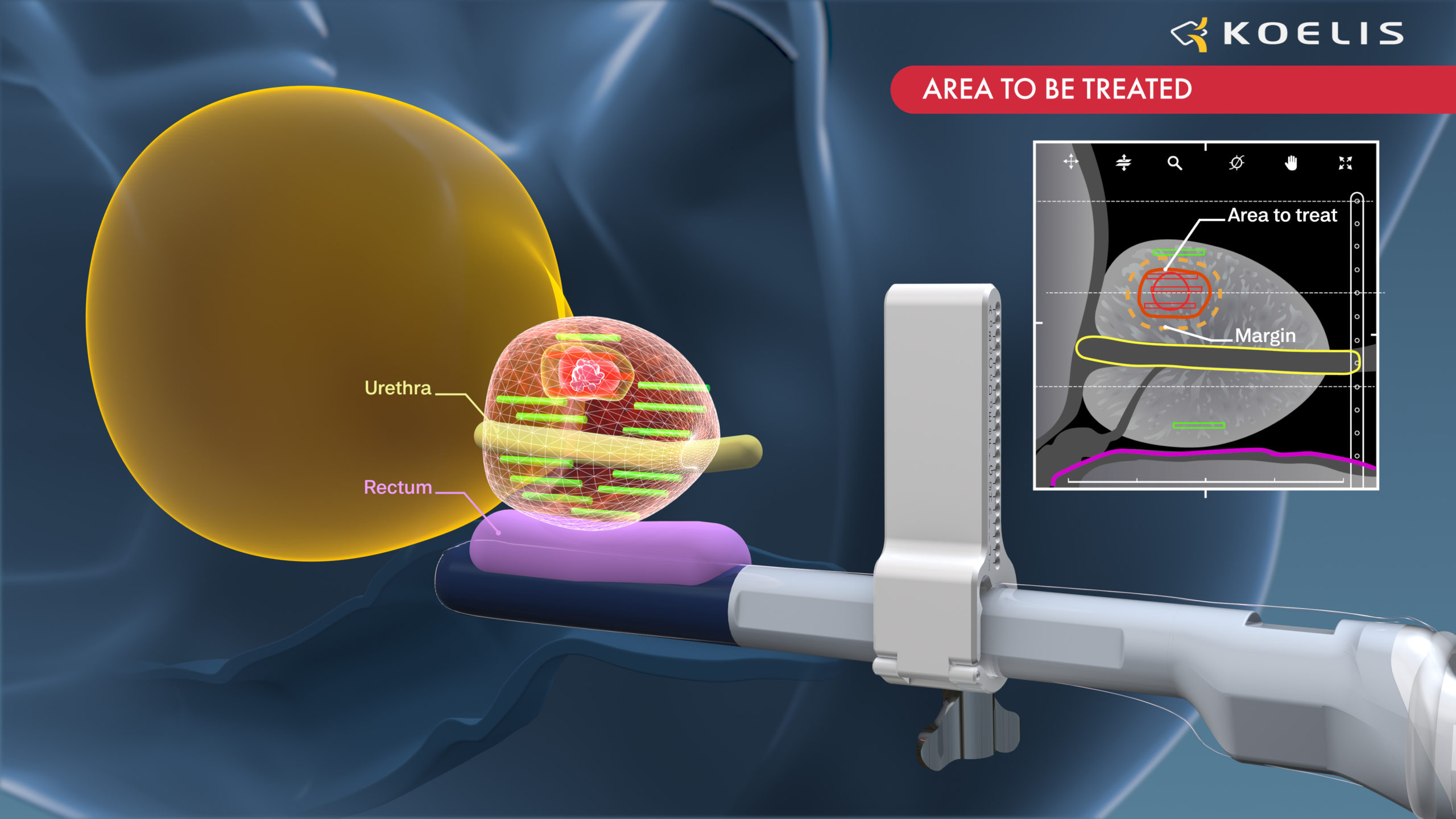
Koelis software fusion interface using 3D ultrasound for transperineal guidance in prostate cancer focal therapy
The VIOLETTE trial assesses the efficacy of focal ablation of prostate cancer using a microwave needle guided by 3D image fusion into the index lesion. The last patient of a total of 76 was successfully enrolled on September 5th, 2024, at the Clinique Saint-Augustin in Bordeaux - FRANCE. Other investigating centers are: Cochin Hospital in Paris as Principal Investigator, Bordet Cancer Institute in Brussels, Urology Clinic Atlantis in Nantes, Pellegrin Hospital in Bordeaux, American hospital Paris.
Interim results were published in BJUI Compass on October 30th, 2024. That analysis focused on treatment data, safety, biological and functional outcomes from the first 37 treated patients.
Key findings include:
- 70% procedures were done transperineally.
- Median pain level, was 0/10 on a VAS 2h after procedure.
- All patients recovered spontaneous micturition and were discharged the same day.
- Over 100% tumor was covered by ablation, DCE-MRI after 7 days showing non-vascularized prostatic tissue in 97% patients.
- A decrease in PSA and PSA density was observed and stable after one month follow-up, indicating early signs of oncological efficacy.
- No significant impact on urinary or sexual function was reported,
- 58 adverse events were reported on 22 patients, including 5 severe resolved at the time of analysis.
Prof. Nicolas Barry-Delongchamps, Professor of Urology at Cochin Hospital and Principal Investigator of the VIOLETTE trial, will present the interim results and early patients follow-up from the VIOLETTE trial at the 118th French Urology Congress in Paris (November 20-23, 2024), where Koelis will exhibit (Booth #B27) and sponsor an educational session on fusion biopsy and focal therapy.
Prof. Barry-Delongchamps commented: “We are delighted to have completed the enrollment of the Violette trial. 76 patients with a single focus of prostate cancer visible on MRI were meticulously selected. The Koelis technology not only helped to detect and characterize these tumors but also allowed precise planning for targeted treatment. As we look forward for the final oncologic results, we believe this new image-guided, needle-based technology is deemed to become an efficient and safe management option for selected patients.”
Prostate cancer remains one of the most prevalent and deadliest cancers among men globally. While recent advances in imaging have contributed to better diagnosis, the Koelis Trinity® system further supports precision care with unparalleled accuracy. Koelis’ technology is supported by over 400 international clinical publications, which underscores the value of OBT-Fusion® for a more accurate and personalized approach in prostate cancer care.
“Prostate care is undergoing a profound shift globally. At Koelis, we believe precision imaging can transform the lives of millions of men. As we work to become the preferred partner for urologists worldwide, we are proud that the VIOLETTE trial demonstrates Koelis’ leading role in enabling focal therapy for prostate cancer,” said Antoine Leroy, PhD, Founder and CEO of Koelis.
About KOELIS
Headquartered in Grenoble, France, Koelis has been a pioneer and leader of MRI-US fusion image guidance technology since 2006. Featuring proprietary 3D ultrasound and prostate motion tracking software (OBT Fusion®), the Koelis Trinity® system facilitates more accurate biopsy diagnosis as well as enabling “focal” prostate cancer treatment alternatives to traditional “total” organ treatments such as surgical prostatectomy and radiation. The Company’s commitment to minimally invasive prostate cancer treatments includes a multi-center clinical registry (“Violette”, NCT04582656) in Europe based on Trinity-guided microwave ablation technology. Koelis has offices in Grenoble (France), Princeton (NJ, USA), Saarbrücken (Germany) and Singapore to serve more than 50 countries. Learn more about KOELIS at www.koelis.com.
Contact presse :
Thomas MARTINS PINHEIRO
Communication Manager
Tél. : + 33 (0)7 88 31 16 48
Thomas.martins-pinheiro@koelis.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/431e3ea9-b7cd-4e23-8038-53ae5bae14e1















