FAYETTEVILLE, Ark. - March 17, 2022 - PRLog -- Lineus Medical signed on its fifth distributor in as many months as it continues to expand its distribution network with the addition of Clinical Technology, a specialty distributor of medical products based out of Brecksville, OH. The Clinical Technology sales team services the mid-western region, covering Illinois, Indiana, Iowa, Kansas, Kentucky, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin. The group employs 14 vascular access sales representatives covering this geographic territory.
"With the addition of Clinical Technology, we now have sales representation across the entire United States, and we are excited to have them as part of the team," Vance Clement, CEO of Lineus Medical stated. "Clinical Technology has a large sales force dedicated to the sales and promotion of vascular access products with unmatched, proven track record in their territory. We are excited to have them on board as a strategic partner."
"Clinical Technology has provided innovative medical devices to hospitals since 1978," said Kent Krafft, Vice President at Clinical Technology. "Our sales force is excited about introducing SafeBreak Vascular to our customers and helping them realize the time savings and cost savings that can be achieved with this product."
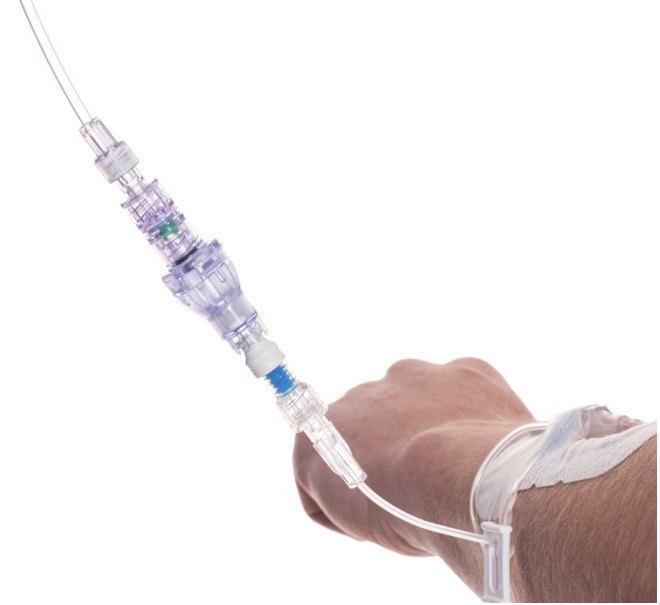
SafeBreak Vascular is a break-away connector that fits into any peripheral IV line in the world. The device is designed to separate when damaging force is placed on an IV line, reducing vein irritation and the chances of the IV dislodging. When the device separates, valves on each side of the device close to stop the flow of medication from the pump and blood flow from the patient's IV catheter. The separated SafeBreak can be replaced with a new, sterile SafeBreak and the IV restarted without the need for another needlestick for the patient.
SafeBreak Vascular reduces the failure of peripheral IV lines and the negative cascade of events that occurs when an IV restart is required. Peripheral IVs fail 46% of the time before their intended use is complete according to the clinical literature and cost the US healthcare system over $5B annually2.
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a patented, break-away technology designed to work with all types of medical tubing. Complications with feeding tubes, IV lines, urinary catheters and other medical tubing may be reduced when a force-activated separation device is used. Learn more at www.lineusmed.com and follow us on LinkedIn or Twitter.
1Data on file.
2Helm, R.E., et al., Accepted but Unacceptable: Peripheral IV Catheter Failure. Journal of Infusion Nursing. 2015
MKG-0131 Rev 00
Photos: (Click photo to enlarge)![]()

![]()
Read Full Story - Lineus Medical Signs Distribution Agreement with Clinical Technology, Covering the Midwest United States | More news from this source
Press release distribution by PRLog
Lineus Medical Signs Distribution Agreement with Clinical Technology, Covering the Midwest United States
March 17, 2022 at 09:01 AM EDT














