TORONTO, ON / ACCESSWIRE / June 20, 2024 / Theralase® Technologies Inc. ("Theralase®" or the "Company") (TSXV:TLT)(OTCQB:TLTFF), a clinical stage pharmaceutical company dedicated to the research and development of light and/or radiation activated small molecules and their formulations, intended for the safe and effective destruction of various cancers, bacteria and viruses, is pleased to announce that in preclinical research, it's lead drug formulation, Rutherrin® was able to repurpose non-cancer drugs for cancer therapy.
Drug repurposing is the process of finding new uses for existing clinically approved drugs.
Repurposing is a drug development strategy that received heightened attention after the Food and Drug Administration ("FDA") granted emergency use authorization of several repurposed drugs to treat the Covid-19 virus. Drug repurposing, defined as researching new indications for already approved drugs or advancing previously studied, but unapproved drugs, is a core approach in drug development. Some reports state that about 30 to 40% of new drugs and biologics approved by the FDA are repurposed or repositioned products, while only 10% of new drug applications achieve approval.
Repurposing drugs has several advantages, such as:
- Cutting research and development costs
- Reducing the drug development timeline
- Reusing drugs that have already demonstrated safety in humans
- Overcoming some of the challenges and knowledge gaps in testing drugs for rare diseases
There is a growing attraction in analyzing off-patent drugs that have established safety, pharmacokinetics (how the human body interacts with a drug) and efficacy and repurposing them for other indications; specifically, cancer, to significantly reduce the cost and time to bring them to market.
Theralase® has demonstrated in preclinical research that Rutherrin® is able to accomplish this task with various drugs, by significantly enhancing their efficacy in the destruction of cancer cells and repurposing their use in the treatment of multiple cancer indications.
Withaferin A, is a steroid primarily used as an anti-inflammatory drug to combat cancer-associated inflammation is being investigated to improve immune checkpoint blockers for cancer treatments.
Amiodarone, an anti-arrhythmic medication used to treat and prevent specific types of cardiac dysrhythmias is being investigated as a cancer treatment.
Metformin, which is the most widely prescribed medicine for type 2 diabetes has been investigated for its potential as an anti-cancer treatment.
Cells were treated with 3 µM Rutherrin®, before addition of each of the drugs mentioned above, to analyze cell survival. As shown in Figure 1 , Rutherrin® significantly increased the cancer cell kill for all tested non-cancer drugs, without light and/or radiation activation, suggesting that Rutherrin® can be combined with these drugs to repurpose them in the destruction of cancer.
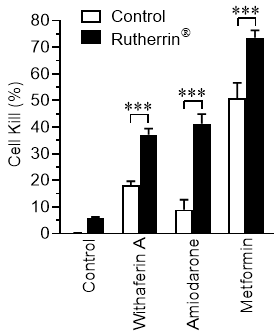
Dr. Arkady Mandel, M.D., Ph.D., D.Sc., Chief Scientific Officer of Theralase® stated, "Despite the tremendous resources being invested in cancer prevention and treatment, cancer remains one of the leading causes of mortality worldwide. Progressively increasing failure rates, high cost, poor bioavailability, poor safety, limited efficacy and a lengthy research and development process associated with cancer drug development has necessitated alternative approaches to drug discovery. During the past decade, interest in finding new uses for old drugs has grown among clinicians and researchers. Using a "lock and key" analogy, our latest preclinical research has demonstrated the ability of Rutherrin® as the "key" to "unlock" approved non-cancer drugs to repurpose them for enhanced anti-cancer activity, literally, "Teaching an old dog, a new tricks." The scientific and clinical rational for this repurposing approach lies in the fact that different diseases share common molecular pathways and targets in the cell. We are excited by the results of our latest research, suggesting that there are a large number of existing non-cancer drugs that can be repurposed for cancer treatment, beyond, what we have initially analyzed, but also that Rutherrin® can significantly increase their efficacy in the targeted killing of cancer cells. This latest Theralase® research provides an opportunity for companies and clinicians to rapidly advance therapeutic strategies into clinical studies and commercialization, using pre-existing approved drugs."
Roger DuMoulin-White, B.E.Sc., P.Eng., Pro.Dir., President and Chief Executive Officer of Theralase® stated, "Most of the initiatives for repurposing existing drugs has stemmed from the multi-billion-dollar cost and decades of time required in today's regulatory environment to research, develop and gain regulatory approval for the safety and efficacy of a new drug. Drug development is long, resource-intensive and highly uncertain as to clinical success, with significant risks along the way and the path to new treatments rarely straightforward. This is why so few companies are able to undertake this journey; except for the much larger and much better financed international organizations. 8 to 12% of drug candidates are successful in graduating from the preclinical phase to FDA approval. It is estimated that a new medicine will take an average of 10 to 15 years and more than $USD 2 billion before it can reach the pharmacy shelf, with an approximate failure rate of 90%. The drug development process has led to significant clinical advances, but the entire process is tedious, time consuming and extremely expensive; therefore, exploring established non-cancer drugs for anti-cancer activity provides a cost and time efficient, de-risked opportunity to rapidly advance therapeutic strategies into clinical studies and commercialization."
About Theralase® Technologies Inc.:
Theralase® is a clinical stage pharmaceutical company dedicated to the research and development of light and/or radiation activated small molecule compounds, their associated drug formulations and the light systems that activate them, with a primary objective of efficacy and a secondary objective of safety in the destruction of various cancers, bacteria and viruses.
Additional information is available at www.theralase.com and www.sedar.com
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward Looking Statements:
This news release contains "forward-looking statements" within the meaning of applicable Canadian securities laws. Such statements include; but, are not limited to statements regarding the Company's proposed development plans with respect to small molecules and their drug formulations. Forward looking statements may be identified by the use of the words "may,", "should", "will", "anticipates", "believes", "plans", "expects", "estimate", "potential for" and similar expressions; including, statements related to the current expectations of Company's management for future research, development and commercialization of the Company's small molecules and their drug formulations, preclinical research, clinical studies and regulatory approvals.
These statements involve significant risks, uncertainties and assumptions; including, the ability of the Company to fund and secure the regulatory approvals to successfully complete various clinical studies in a timely fashion and implement its development plans. Other risks include: the ability of the Company to successfully commercialize its small molecule and drug formulations, the risk that access to sufficient capital to fund the Company's operations may not be available on terms that are commercially favorable to the Company or at all, the risk that the Company's small molecule and drug formulations may not be effective against the diseases tested in its clinical studies, the risk that the Company's fails to comply with the terms of license agreements with third parties and as a result loses the right to use key intellectual property in its business, the Company's ability to protect its intellectual property, the timing and success of submission, acceptance and approval of regulatory filings. Many of these factors that will determine actual results are beyond the Company's ability to control or predict.
Readers should not unduly rely on these forward-looking statements, which are not a guarantee of future performance. There can be no assurance that forward-looking statements will prove to be accurate as such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause actual results or future events to differ materially from the forward-looking statements.
Although the forward-looking statements contained in the press release are based upon what management currently believes to be reasonable assumptions, the Company cannot assure prospective investors that actual results, performance or achievements will be consistent with these forward-looking statements.
All forward-looking statements are made as of the date hereof and are subject to change. Except as required by law, the Company assumes no obligation to update such statements.
For investor information on the Company, please feel to reach out Investor Inquiries - Theralase Technologies.
For More Information:
1.866.THE.LASE (843-5273)
416.699.LASE (5273)
www.theralase.com
Kristina Hachey, CPA
Chief Financial Officer
X 224
khachey@theralase.com
SOURCE: Theralase Technologies Inc.
View the original press release on accesswire.com













