
Sarepta Therapeutics Inc. (NASDAQ: SRPT) is a leading commercial-stage biotech focused on developing precision gene therapy treatments for rare diseases. This medical sector company specializes in the treatment of Duchenne muscular dystrophy (DMD), a congenital myopathy that occurs in one in 3,600 male live-born infants in the United States.
The company has started generating revenues for its fourth DMD treatment, called ELEVIDYS, which received accelerated approval in June 2023 for children ages four to five with a confirmed mutation of the DMD gene.
One treatment: $3.2 million
Sarepta charges $3.2 million for a one-time ELEVIDYS gene therapy to get the muscles to produce ELEVIDYS micro-dystrophin. Sarepta has a pipeline of over 40 programs in various research stages for treating DMD and rare genetic diseases like limb-girdle muscular dystrophy type 2E (LGMD2E).
The company partners with Roche Holding AG (OTCMKTS: RHHBY) for European distribution and competes with Pfizer Inc. (NYSE: PFE) and Wave Life Sciences Ltd. (NASDAQ: WVE) in gene therapies for DMD.
ELEVIDYS accelerated FDA approval is narrow
ELEVIDYS (SRP-9001) was the first gene therapy approved for DMD by the FDA on June 22, 2023. It was granted accelerated approval for ambulatory pediatric patients with a confirmed mutation in the DMD gene.
It was a narrow label since the studies also included children ages six to seven, which showed less conclusive results. The accelerated approval is not a full approval, which requires subsequent studies to receive full approval. Accelerated approvals fast-track drugs for serious conditions filling an unmet medical need and approved based on a surrogate endpoint.
Phase 3 EMBARK study
On October 31, 2023, the results of its Phase 3 EMBARK multinational clinical study for DMD children aged four to seven failed to reach its primary endpoint of statistical significance in the confirmatory study, despite the company's spin and intent to ask for label expansion.
The results caused shares to plummet 42%. The company disregarded this and still pursues full approval despite missing the primary endpoint. Since they are the only FDA-approved DMD gene therapy treatment, the unmet need for ELEVIDYS is too high to limit availability despite the lower significance of its therapy for kids ages six to seven.
ELEVIDYS BLA efficacy supplement
Sarepta filed an efficacy supplement to its Biologics License Application (BLA) to expand the label for ELEVIDYS on December 22, 2023. The company stated in its Q3 2023 earnings report press release, "ELEVIDYS-treated patients showed an increase on the North Star Ambulatory Assessment, a measure of motor function, compared to placebo-treated patients at 52 weeks, although the primary endpoint was not met."
The statement continued, "Robust, statistically significant results on all key functional pre-specified secondary endpoints, including time to rise (p=0.0025), and 10-meter walk test (p=0.0048), demonstrated evidence of a clinically meaningful treatment benefit that was similar in magnitude and statistical significance across all age groups."
Sarepta CEO Doug Ingram is so confident that the FDA will expand the label despite its Phase 3 results that he predicted the restriction would be lifted in months, not years, and he expects full approval by August 2024. Shares have since recovered from its October 31 stock collapse.
Pipeline
As noted earlier, Sarepta has over 40 programs in various stages of research. SRP-9003 is a late-stage gene therapy for LGMD2E. The company has also teamed with Harvard University and Duke University on gene-editing therapy discovery programs using CRISPR/Cas9 technology. SRP-5051 is a late-stage RNA-targeted therapy for DMD.
Check out the sector heatmap on MarketBeat.
Solid Q3 2023 earnings report
Sarepta reported non-GAAP earnings of 37 cents per share in Q3 2023. Revenues rose 44.1% to $331.8 million, crushing analyst estimates for $285.33 million.
Net product revenue rose 49% to $309.32 million, driven by EXONDYS, VYONDYS and AMONDYS treatments and ELEVIDYS generating $69.11 million, nearly triple the mean external consensus. Get AI-powered insights on MarketBeat.
Preliminary Q4 2023 guidance raise
On January 8, Sarepta announced a total 2023 net product revenue of $1.145 billion. Year-end cash and cash equivalents were $1.7 billion. ELEVIDY net product revenues for $131.3 million for Q4 2023 and $200.4 million for full-year 2023, significantly exceeding consensus. Preliminary RNA-based PMO net product revenues are expected to be $243.3 million and $945 million for Q4 and the full year of 2023, respectively, versus $925 million in previous guidance.
Sarepta Therapeutics analyst ratings and price targets are at MarketBeat. You can find Sarepta Therapeutics' peers and competitor stocks with the MarketBeat stock screener. SRPT has a 5% short interest.
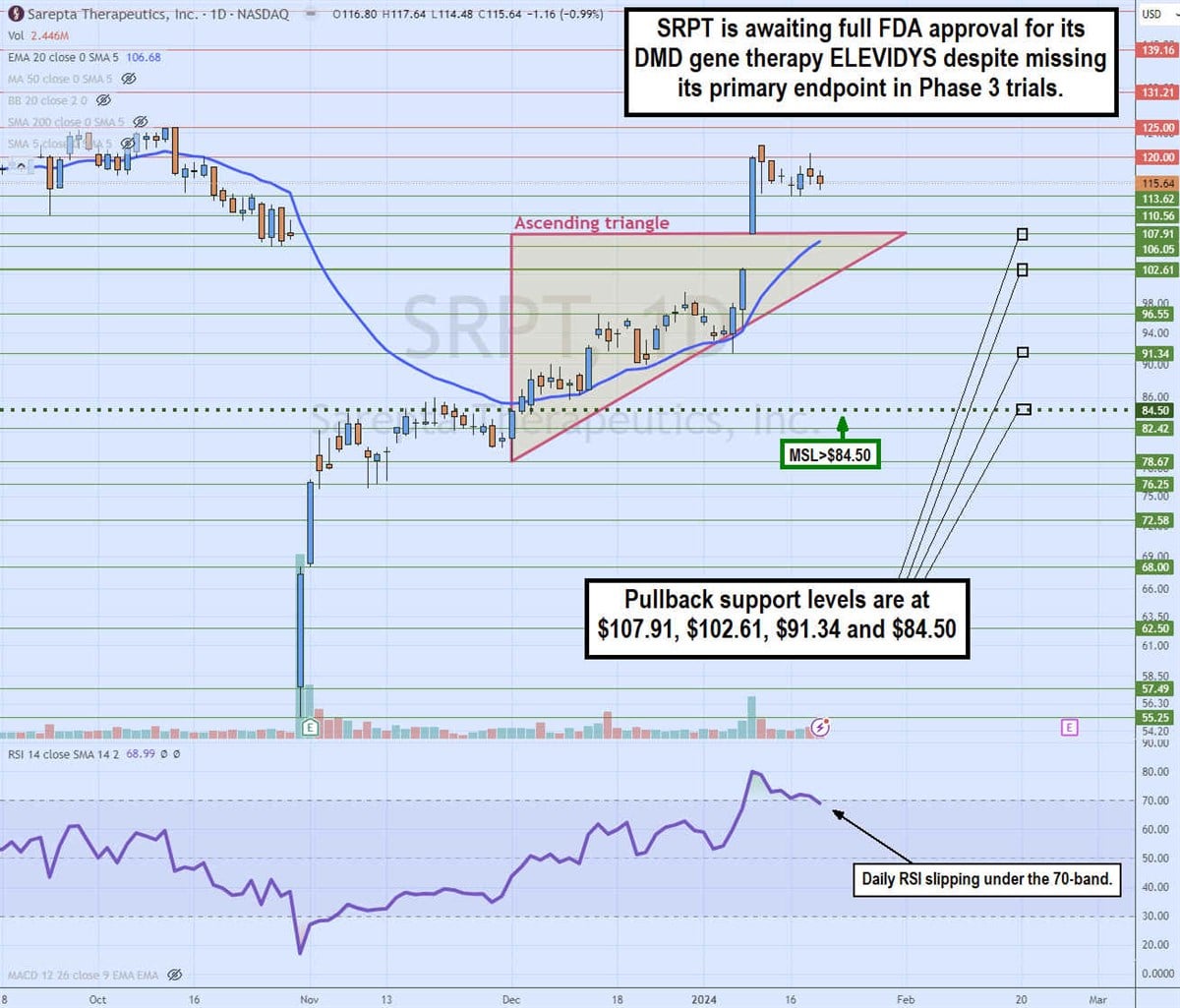
Daily ascending triangle breakout
The daily candlestick chart on SRPT illustrates a daily ascending triangle breakout pattern. Shares collapsed over 40% after reporting results of its Phase 3 EMBARK study on October 31, bouncing off a low of $55.25. The following day, its Q3 2023 earnings report helped to stage a rally through its daily market structure low (MSL) trigger at $84.50. SRPT bounced up through its daily 20-period exponential moving average (EMA) at $85.31 as the resistance became a solid rising support.
The ascending trendline formed at $78.67 and formed higher lows on pullbacks assisted by the 20-period EMA acting like a trampoline. The flat-top horizontal upper trendline at $107.91 breakout occurred on a gap on a January 9, preliminary Q4 2023 update, shooting shares up to a high of $121.91 before the reversion formed on the daily relative strength index (RSI) slipping under the overbought 70-band.
The pullback support levels are $107.91, $102.61, $91.34 and $84.50. If the FDA doesn't expand the label with full approval, then SRPT shares could experience a price collapse.













